Cochlear implants are electronic hearing devices implanted into people with severe to profound hearing loss to produce useful hearing sensations. They have made a profound contribution to the quality of life for many people, especially children. Today, the FDA has issued a Public Health Notification concerning continued risk, beyond the twenty-four months post-implantation period described in previous reports, of bacterial meningitis in children with some of these cochlear implants.
The FDA has provided the following advice for people with cochlear implants.
Advice for Patients With Cochlear Implants: New Information on Meningitis Risk
February 6, 2006
This advice for patients gives the latest information on the risk of bacterial meningitis in children with cochlear implants and recommends steps to reduce the risk.
Previous Information
A study published in 2003 (NewEngJMed, 349.5: 435-445) (http://content.nejm.org/cgi/content/full/349/5/435) by FDA and the Centers for Disease Control and Prevention (CDC) followed children with cochlear implants for two years after the device was implanted. The study showed that children whose implants have a positioner get bacterial meningitis more often than children with implants that don’t have positioners or children without implants. A positioner is a small rubber wedge that helps the physician position the implant during surgery. Bacterial meningitis, a serious infection in the cerebrospinal fluid (CSF) around the brain and spinal cord, can be fatal. Only Advanced Bionics Corporation sold an implant that had a positioner, and none were implanted after July 2002.
New Information
Now a new study (Pediatrics February 2006), which followed the children for two more years, has found that the increased risk for meningitis persists beyond two years after implantation. This study highlights the importance of continuing to monitor children with cochlear implants for signs of middle ear infection and meningitis. Children need to be monitored for as long as the implant is in place.
Recommendations
- Continue close monitoring for meningitis and middle ear infections for all children with a cochlear implant, but particularly for children whose implants have a positioner. Consult your implanting doctor to determine if the patient has a positioner. It is, however, important to monitor all cochlear implant patients.
- Check the patient’s record of vaccinations against CDC’s recommendations (www.cdc.gov/nip/issues/cochlear/cochlear-gen.htm), which show what vaccines cochlear implant patients should receive and when the vaccines should be given. The CDC Immunization Center telephone number is 800-232-4636. The TTY number is 888-232-6348.
- Contact your doctor immediately if the patient has any symptoms of meningitis or middle ear infection. These may include:
• high fever • discomfort looking into bright lights • headache • sleepiness or tiredness • stiff neck • confusion • nausea • ear pain • vomiting • hearing loss • irritability • appetite loss
- Follow your doctor’s prescription for antibiotics very carefully. It is very important that you make sure the patient takes the antibiotic as often as prescribed and for as long as prescribed, so it can work properly.
Additional Background Information
The original CDC/FDA study reviewed the medical records of 4,264 children under the age of six at the time of implantation. The study was undertaken because of increased concern about the risk of meningitis associated with cochlear implants. The study focused on young children because they account for most known meningitis cases, and they represent the population that now receives a large proportion of cochlear implants
In the original study, 26 of the 4,264 children developed meningitis during the first 24 months after implantation. Children who had cochlear implants with electrode positioners developed meningitis more often than children who had implants without positioners. The study was unable to determine how the positioner increased the risk for developing meningitis. Because the number of meningitis cases in the original study was small, it is hard to predict the risk of developing meningitis with various cochlear implant models.
The new study followed children from the original study for an additional two years. Six children with positioners developed meningitis after two years. Of these six, three developed meningitis between three and four years after implantation. Children in the study without a positioner did not develop meningitis, but this group of children was so small it is hard to predict the risk of their developing meningitis. The study also concluded there is not enough information now to recommend surgical removal of devices with a positioner.
Information for health care providers appears on the web at http://www.fda.gov/cdrh/safety/020606-cochlear.html.
For general information on cochlear implants visit:
http://www.fda.gov/cdrh/cochlear/
I do recommend working closely with the surgeon who performed the implant and I appreciate the rationale behind both the vaccination schedule and the liberal attitude towards antibiotic use. However, I do not agree with the orthodox implementation of either of these.
Vaccinations are included as preventative measures, yet the range and severity of harm caused by vaccination significantly outweighs the supposed but questionable benefits in my opinion, in most situations. There are better ways to promote a healthy and vigorous immune system.
Equally, pharmaceutical antibiotics cause more harm than good and doctors competent in their use are very rare indeed. For instance, when you were last prescribed antibiotics was there a part of the treatment plan explained to you involving follow-up with probiotics to undo some of the "collateral damage" caused by the treatment? And was it implemented? If the answers are yes, congratulations, you probably have found a naturopathic doctor or at least an enlightened MD.
Antibiotic coverage can be provided by natural antibiotics. I suggest you consult a naturopathic doctor and ask your surgeon to liaise with this practitioner for your ongoing management.
I do recommend that general information link above, provided by the FDA. It has some quite useful information, including links helpful for teachers of children with cochlear implants.
I have recommended against the consumption of dairy products on the grounds that they undermine health. Yes, that’s right, undermine, as opposed to the marketing spin that would have you believe dairy products build health, or at minimum "healthy teeth and bones". The marketers have it wrong.
It seems the message is increasingly finding exposure in the normal press. For example, the following was written by Julie Deardorff for the Chicago Tribune 5 Febreary, 2006. It was titled: "not milk?"
If you can’t imagine life without a daily dose of dairy, consider new research that questions the value—-if not the safety—-of this dietary staple
You know it like the Pledge of Allegiance: "Milk helps build strong teeth and bones."
But does it really? Or, as nutrition researchers from Harvard and Cornell Universities are radically suggesting: Have we all been duped by the dairy industry’s slick, celebrity-driven "got milk?" advertising campaign?
Milk, the sacred cow of the American diet, is under attack and not just by animal-rights activists. Though federal dietary guidelines and most mainstream nutrition experts recommend that people age 9 or older drink three glasses of milk a day, researchers are examining the role of dairy in everything from rising osteoporosis rates, Type 1 diabetes and heart disease to breast, prostate and ovarian cancer.
Last March, the journal Pediatrics published a review article concluding that there is "scant evidence" that consuming more milk and dairy products will promote child and adolescent bone health. Some leading practitioners of integrative medicine, including best-selling author Dr. Andrew Weil, suggest eliminating dairy products from the diet to help treat irritable bowel syndrome, asthma, eczema and ear infections. The late Dr. Benjamin Spock reversed his support of cow’s milk for children in 1998 in his last edition of his world-famous book "Baby and Child Care."
One fact is indisputable: Our bodies need the mineral calcium to build and maintain bones and teeth. Calcium also helps with blood clotting, muscle function and regulation of the heart’s rhythm. The debate centers on whether milk is really the best–or even a necessary–source. Ten thousand or so years ago, cow’s milk was not part of the human diet.
Whom do you believe?
For consumers, the issue is profoundly confusing, especially when it comes to osteoporosis. On one hand, we’ve had it hammered home since grammar school that milk is a health food. We’re told that increasing calcium intake by drinking milk will prevent osteoporosis, the weakening of bones.
But researchers Walter Willett, chairman of the department of nutrition at the Harvard School of Public Health, and T. Colin Campbell, professor emeritus of nutritional biochemistry at Cornell University, say there is little evidence that shows boosting your calcium intake to the currently recommended levels will prevent fractures.
Willett, who co-authored "The Nurses’ Health Studies," one of the largest investigations into the risk factors for major chronic diseases in women, found that women with the highest calcium consumption from dairy products actually had substantially more fractures than women who drank less milk.
Campbell, who like Willett comes from a dairy-farming family, found the same thing after spending several decades surveying health-related effects of a plant-based diet and death rates from cancer in more than 2,400 Chinese counties.
Both men say there is no calcium emergency; Americans get plenty. And they argue that the unnecessary focus on calcium prevents us from using strategies that really work in the fight against osteoporosis, including getting enough exercise and vitamin D and avoiding too much vitamin A.
"The higher the consumption of dairy, animal protein and calcium, the higher the fracture rate–an indisputable observation in my view," said Campbell, whose life work is compiled in "The China Study" (Benbella Books, $24.95), one of the most comprehensive nutritional studies undertaken.
The link between milk and cancers is sketchier–peer-reviewed studies back both pro- and anti-dairy viewpoints–though a growing body of evidence has shown that animal-based foods are associated with prostate cancer, possibly because of the high intake of calcium and phosphorus, Campbell said.
The dairy industry, the federal government and most conventional registered dietitians and nutritionists say just the opposite. Milk is more than just calcium; it’s a relatively cheap little package of fat, vitamins, proteins, carbohydrates and minerals.
Some research shows calcium may help protect against colon cancer and high blood pressure. A large-scale government study called DASH (Dietary Approaches to Stop Hypertension) found that a balanced, low-fat diet rich in fruits, vegetables and low-fat dairy foods may help reduce blood pressure as effectively as some medications.
The calcium from some vegetables such as broccoli, bok choy and kale is absorbed as well as or better than calcium from milk and milk products, according to the National Dairy Council’s Calcium Counseling Resource. But the report also says that to get the same amount of calcium absorbed from 1 cup of milk, one would have to eat nearly 2 1/2 cups of broccoli or 8 cups of spinach.
"The advantage of dairy is that it’s convenient, and children are more likely to consume it over broccoli and prunes," said Jeanette Newton Keith, a gastroenterologist at the University of Chicago. She advocates a whole-food diet and recommends dairy as part of the DASH plan.
"Anti-dairy groups say you don’t need it in the diet. Unfortunately, 83 percent of the calcium in our diets comes from dairy foods," Keith said.
Though dairy is high in saturated fat, the dairy industry claims that low-fat dairy products can encourage weight loss. During the last few years it has spent millions on a controversial "got milk?" advertising campaign, using milk-mustachioed figures such as television’s Dr. Phil McGraw.
In response, the Physicians Committee for Responsible Medicine (PCRM) filed false-labeling petitions last June with the Federal Trade Commission and the Food and Drug Administration. They maintain that the "got milk?" weight-loss ads are "dishonest," because scientific evidence contradicts the claims. The dairy industry based its assertion largely on the work of University of Tennessee researcher Michael Zemel, who received funding from the Dairy Council and who also has patented a weight-loss program using calcium.
(The PCRM, which includes about 5,000 physicians among its 100,000 members, is often accused of "having ties" with People for the Ethical Treatment of Animals [PETA]. In fact, it’s a separate organization from PETA and separately funded, though the groups have similar ideologies, said Amy Joy Lanou, PCRM’s senior nutrition scientist and the author of the review in Pediatrics that found that milk products didn’t necessarily promote bone health in children.)
An overlapping focus
"Our work promoting preventive medicine through healthy eating–with a focus on a plant-based diet–does overlap with PETA’s work in the sense that they also are promoting vegetarian and vegan diets and compassionate living," said Lanou, an assistant professor of nutrition in the department of health and wellness at the University of North Carolina.
For Mickey Hornick of Chicago, showing kindness toward other creatures was the reason he began considering a dairy-free lifestyle. But he eventually turned vegan for health reasons.
"I was often congested and had asthma-like symptoms," he said. "When I removed all dairy from my diet, my breathing greatly improved without any medication."
Selling soy products
Hornick and his wife, chef Jo Kaucher, who co-own the meat-free restaurant Chicago Diner, have found a growing market for their soy cheeses (casein-free), soy, rice and nut milks, organic soy ice creams, vegan cream cheese and tofu ricotta.
They send dairy-free cookies, muffins and cheesecakes to 18 Whole Foods stores across the Midwest and other local stores and restaurants, including Wild Oats grocery stores, Evanston’s Blind Faith, Argo Teas, Chicago’s Kopi Cafe and Uncle Joe’s at the University of Chicago.
The restaurant has been an oasis for Chicago’s Rikke Vognsen and her husband, David Saxner, who cut dairy out of their diets 20 years ago to help with Saxner’s arthritis. He also lost 80 pounds in the process. Their belief is that dairy creates dampness in the body and promotes yeast growth. But they also wanted to avoid ingesting residues of the hormones, antibiotics and other supplements given to the cows that produce non-organic milk.
(The dairy industry contends that milk is free of antibiotics given to cows and that there is no "significant" difference in cows treated with hormones produced with biotechnology, known as recombinant bovine somatotropin [rbST]).
"We saw immediate improvements in my husband’s health after eliminating dairy," Vognsen said.
But some can’t imagine life without whole milk in their lattes or mozzarella cheese on their pizza. Chicago’s Trina Kakacek, the adult aquatic director at Lakeshore Athletic Club Lincoln Park, drinks a glass of skim milk and eats cheese and yogurt daily. Once a week she treats herself to ice cream.
"I would never dream of giving up dairy, particularly cheese or the real cream in my coffee every morning," said Kakacek, who is allergic to nuts and soy and rarely eats meat.
Make vegetables’ calcium more palatable
No one ever said eating a fennel bulb or beet green would be as much fun as a slice of pepperoni pizza or a big scoop of chocolate ice cream, but a few simple steps will make calcium-rich vegetables taste better.
Buy in-season and buy the best quality you can afford. Just-picked vegetables in peak condition have more flavor than any tired old things trucked in from California two weeks ago. If that means shifting gears on your meal plan at the market, so be it.
Don’t overcook vegetables. Think of those crisp-yet-tender veggies found in classic Chinese stir-fries.
Vary your presentation. You’ve served steamed vegetables every week for the last six months; do something different. Puree for soup (cooked rice can be whooshed in the food processor or blender with the vegetables to give the soup a creamy texture). Chop fine and fold into an omelet. Saute them in olive oil with garlic, lemon zest and some red pepper flakes. Experiment.
Remember to season as you cook, especially when it comes to salt and pepper. Taste every step of the way and adjust flavorings accordingly.
Get out of the butter rut. Accent your cooked vegetables with an eye toward flavor, texture and color. A sprinkling of minced red onion and a shot of orange juice do wonders for fennel. A spritz of lemon works for bok choy. Chopped walnuts pair well with greens like kale. And minced sauteed garlic perks up any vegetable.
I was very interested to note the following paragraph above.
… the Physicians Committee for Responsible Medicine (PCRM) filed false-labeling petitions last June with the Federal Trade Commission and the Food and Drug Administration. They maintain that the "got milk?" weight-loss ads are "dishonest," because scientific evidence contradicts the claims. The dairy industry based its assertion largely on the work of University of Tennessee researcher Michael Zemel, who received funding from the Dairy Council and who also has patented a weight-loss program using calcium.
This is very reminiscent of the action taken last year in the U.K. where the Vegan Society complained to the UK Advertising Standards Authority (ASA) about an advertorial sponsored by Nestle published on an AOL website and won their case. The ASA found that claims in the advertorial that implied milk was essential to obtain healthy bones was misleading.
Yes, we need calcium and the other good ingredients regularly listed as being provided by milk. However, dairy products are not the healthy way to obtain those nutrients and the dairy products include substances which cause harm. Dairy products may be loved by many, obviously be crucial to the dairy industry and have been promoted by health authorities for many years but modern science and epidemiology are telling us what naturopaths have been saying for a very long time: dairy foods are not health products.
The Food and Drug Administration (FDA) today announced the approval of RotaTeq(tm), a live, oral, vaccine for use in preventing rotavirus gastroenteritis in infants. It is the only vaccine approved in the United States that can help protect against rotavirus, a viral infection that may cause diarrhea, vomiting, fever, and dehydration.
"This vaccine gives health care providers an important new tool that can effectively prevent an illness that affects almost all children within the first few years of life," said Jesse L. Goodman, MD, MPH, director of FDA’s Center for Biologics Evaluation and Research. RotaTeq(tm) is a liquid vaccine that is given by mouth in three doses, between the ages of 6 and 32 weeks.
Infection with rotavirus is a leading cause of severe diarrhea in infants and young children in the United States and worldwide. The Centers for Disease Control and Prevention (CDC) has estimated that rotavirus infection results in approximately 55,000 hospitalizations annually of infants and young children in this country. Death from rotavirus is rare in the United States. However, in developing countries, rotavirus gastroenteritis has been estimated to cause up to several hundred thousand deaths annually in infants and young children.
Overall, approximately 72,000 healthy infants were studied in the United States and other countries in randomized placebo-controlled studies to look at the safety of RotaTeq(tm). Of these infants, almost 7,000 from the United States and Finland were also studied for efficacy. In these studies, RotaTeq(tm) prevented 74 percent of all rotavirus gastroenteritis cases and 98 percent of the severe cases. In addition, RotaTeq(tm) prevented approximately 96 percent of hospitalizations due to rotavirus gastroenteritis.
In 1998, the FDA got it wrong when it approved a different live vaccine against rotavirus that was later withdrawn from the market because of its association with an increased risk of intussusception, a rare, life-threatening type of blockage or twisting of the intestine. Intussusception occurs spontaneously in approximately 1 in 2,000 healthy young infants and children per year, but occurred at an increased rate during the first week or two following vaccination with the previous rotavirus vaccine.
The risk of intussusception for RotaTeq(tm) was evaluated in a large-scale trial of over 70,000 children, of whom half received vaccine and the remaining half received placebo. In this study, RotaTeq(tm) was not associated with an increased risk of intussusception when compared to placebo.
The FDA asserts that "RotaTeq(tm) was not associated with an increased risk of other serious adverse events when compared to placebo." However, it acknowledges that the following were reported more often in infants who received RotaTeq(tm) when compared to infants who received placebo:
- diarrhea (24.1 percent in vaccine recipients vs 21.3 percent in those receiving placebo),
- vomiting (15.2 percent in vaccine recipients vs 13.6 percent in those receiving placebo),
- ear infection (14.5 percent in vaccine recipients vs 13.0 percent in those receiving placebo),
- runny nose and sore throat (6.9 percent in vaccine recipients vs 5.8 percent in those receiving placebo),
- wheezing and coughing (1.1 percent in vaccine recipients vs 0.7 percent in those receiving placebo).
The FDA understandably is sensitive to the possibility of another failure like its last approved rotavirus vaccine and virtually admits that consumers will be relegated to the role of research subjects when Dr. Goodman says: "Although this large study did not show an increased risk of intussusception associated with RotaTeq(tm), given the experience with the previous vaccine, safety of this vaccine will be closely monitored in additional studies conducted after licensure." The manufacturer, Merck & Co., Inc., has committed to conducting a post-licensure study of approximately 44,000 children. CDC will also conduct a large study designed to rapidly detect any association of intussusception with RotaTeq(tm) through its Vaccine Safety Datalink Program, which evaluates vaccine safety in approximately 80,000 U.S. infants every year. In addition, for the first three years of licensure, the manufacturer will report cases of intussusception and all serious and unexpected adverse events to FDA within 15 days of receiving them, and all other side effects on a monthly basis.
Reducing the incidence of rotavirus gastroenteritis and the risks it poses for poor outcomes is an understandable and desirable motive. However, vaccination is not the way to go, especially not mass vaccination. In time we will see a clearer picture of the unwanted side effects associated with the vaccine, there is nothing more certain. This is sad and unfortunate.
Under its comprehensive framework for ensuring the safety of human tissue products, the U.S. Food and Drug Administration (FDA) today ordered Biomedical Tissue Services, Ltd. (BTS), of Fort Lee, NJ, a human tissue-recovery firm, and its CEO and Executive Director of Operations, Michael Mastromarino, D.D.S., to immediately cease all manufacturing operations. All tissue products initially recovered from human donors by BTS were recalled. FDA is carefully monitoring these recalls to account for all of the tissue distributed.
"FDA’s investigation of BTS revealed serious and widespread deficiencies in their manufacturing practices that provide the agency reason to believe that allowing the firm to manufacture would present a danger to public health by increasing the risk of communicable disease transmission," said Margaret O’K. Glavin, FDA’s Associate Commissioner for Regulatory Affairs.
"FDA’s current regulatory framework for Human Tissue and Cellular and Tissue Based Products (HCT/Ps) provides strong measures that the agency can utilize to prevent the introduction, transmission, or spread of communicable diseases by HCT/Ps, and require firms to screen and test donors for relevant communicable disease agents and diseases and to ensure that HCT/Ps are processed in a way that prevents communicable disease contamination and cross-contamination," added Jesse L. Goodman, MD, MPH,
director of FDA’s Center for Biologics Evaluation and Research.
The FDA order to cease manufacturing and to retain HCT/Ps requires BTS to suspend any and all manufacturing steps, including but not limited to the recovery and shipment of HCT/Ps. FDA’s inspection of BTS uncovered serious violations of the regulations governing donor screening and record keeping practices, as well as failures to follow their own standard operating procedures (SOPs), failure to recover HCT/Ps in a manner that does not cause contamination or cross-contamination during recovery, and failure
to adequately control environmental conditions.
Despite records maintaining otherwise, the firm had inadequately screened donors for risk factors for, or clinical evidence of, relevant communicable disease agents and diseases. In addition, FDA found numerous instances where death certificates maintained in BTS’ files were at variance with the death certificates FDA obtained from the state where the death occurred, on important information such as cause, place, and time of death, and the identity of the next of kin.
For example, the official Cease order documented the following irregularities, along with many others.
- You confirmed the eligibility of donor ————, who donated HCT/Ps at a funeral home in ———-. The donor is listed on the BTS version of the certificate of death as being 63 years of age, having died of acute myocardial infarction due to coronary artery disease, at —-pm on ————, whereas the State of —————issued certificate of death lists this donor as being 69 years of age, having died of multi-organ failure, due to liver dysfunction, which in turn was due to thrombosis, at —-pm on ——————;
- You confirmed the eligibility of donor ————–, who donated HCT/Ps at a funeral home in —————-. The donor is listed on the BTS version of the certificate of death as being 44 years of age, having died of blunt trauma in a motor vehicle accident, at —-am on —————-, whereas the State of —————–issued certificate of death lists this donor as being 48 years of age, having died of congestive cardiac failure due to atherosclerotic cardiovascular disease, at —-am on ——————;
- You confirmed the eligibility of donor ————-, who donated HCT/Ps at a funeral home in —————. The donor is listed on the BTS version of the certificate of death as being 70 years of age, having died of cardio-pulmonary arrest due to acute myocardial infarction, at —- pm on ——————, whereas the State of ———————issued certificate of death lists this donor as being 74 years of age, having died of complications from the intravenous administration of medication due to a radical resection of a ——————————————, at —- pm on ———————;
- You confirmed the eligibility of donor ——————, who donated HCT/Ps at a funeral home in —————. The donor is listed on the BTS version of the certificate of death as having died of cardio-pulmonary arrest due to atherosclerotic heart disease, at —-pm on —————–, whereas the State of ————-issued certificate of death lists this donor as having died of cardio-pulmonary asystole due to sepsis and shock, at —-pm on ————–;
- You confirmed the eligibility of donor —————, who donated HCT/Ps at a funeral home in ———————-. The donor is listed on the BTS version of the certificate of death as being 45 years of age, having died of blunt trauma due to a motor vehicle accident, at —-pm on —————, whereas the State of ———————-issued certificate of death lists this donor as being 41 years of age, with a cause of death which was undetermined pending additional studies, at —-pm on ——————-;
- You confirmed the eligibility of donor ————-, who donated HCT/Ps at a funeral home in ————-. The donor is listed on the BTS version of the certificate of death as having died of cardio-pulmonary arrest due to acute myocardial infarction, at —-pm on —————–, whereas the State of ——————issued certificate of death lists this donor as dying of probable ventricular fibrillation due to ——————- failure, as a consequence of ——————– disease, at —-am on ———————-;
- You confirmed the eligibility of donor ————-, who donated HCT/Ps at a funeral home in ————. The donor is listed on the BTS version of the certificate of death as being 58 years of age, having died of acute myocardial infarction, at —-am on ———-, whereas the State of ———————issued certificate of death lists this donor as being 50 years of age, having died of diabetes mellitus and hypertension due to cardio-vascular disease, at —-pm on —————————; and
- You confirmed the eligibility of donor ————–, who donated HCT/Ps at a funeral home in —————-. The donor is listed on the BTS version of the certificate of death as being 63 years of age, having died of acute myocardial infarction, at —-pm on ————, whereas the State of ————-issued certificate of death lists this donor as being 79 years of age, having died of pneumonia due to a myocardial infarction, at —-pm on —————–.
After initially focusing efforts on assessing the safety of distributed tissues and facilitating the appropriate recalls, the Agency has determined that these violations, because of their serious nature, constitute a danger to health and is taking this unprecedented action.
FDA continues to investigate BTS’ activities and to work cooperatively with tissue processors and appropriate federal, state and local authorities, and will take further actions as needed.
You can view a copy of the BTS Order of Cessation at: http://www.fda.gov/cber/compl/bts013106.htm It is certainly interesting. Once again it would appear that the FDA has actually performed a service to consumers, in which case I applaud their intervention. Of course leopards don’t change their spots so I would want to hear from Michael Mastromarino, D.D.S., CEO and Executive Director of Operations of the affected company, before rushing to judgement. For example, I wonder if companies somehow in favor with the FDA are particularly advantaged by this action. Still, on the surface it appears that congratulations to the FDA are in order.
The following article illustrates the detailed monitoring and reporting conducted by the Centers for Disease Control and Prevention (CDC). In this case it describes one week’s worth of monitoring of influenza in the United States. Note that, all the hype notwithstanding, the fact remains that no human avian influenza A (H5N1) virus infection has ever been identified in the United States. However, many deaths do occur every year due to human influenza viruses, and while they are tracked and recorded assiduously, there is a distinct absence of hype surrounding them.
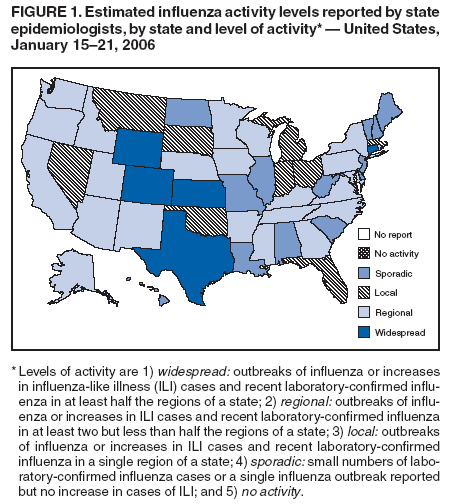
During January 15 to 21, 2006,* the number of states reporting widespread influenza activity†decreased to five. Twenty-three states reported regional activity, nine reported local activity, and 13 reported sporadic activity. This is shown in Figure 1.§
The percentage of specimens testing positive for influenza increased in the United States overall. Since October 2, 2005, the largest numbers of specimens testing positive for influenza have been reported from the Mountain (919 positives) and Pacific (684 positives) regions, accounting for 30.6% and 22.8%, respectively, of positive tests reported during the 2005–06 influenza season. The percentage of outpatient visits for influenza-like illness (ILI)¶ increased during the week ending January 21 and is above the national baseline.** The percentage of deaths attributed to pneumonia and influenza (P&I) was below the epidemic threshold for the week ending January 21.
Laboratory Surveillance
During January 15–21, World Health Organization (WHO) collaborating laboratories and National Respiratory and Enteric Virus Surveillance System (NREVSS) laboratories in the United States reported testing 2,283 specimens for influenza viruses, of which 247 (10.8%) were positive. Of these, 81 were influenza A (H3N2) viruses, 159 were influenza A viruses that were not subtyped, and seven were influenza B viruses.
Since October 2, 2005, WHO and NREVSS laboratories have tested 50,688 specimens for influenza viruses, of which 3,000 (5.9%) were positive. Of these, 2,904 (96.8%) were influenza A viruses, and 96 (3.2%) were influenza B viruses. Of the 2,904 influenza A viruses, 1,388 (47.8%) have been subtyped; 1,381 (99.5%) were influenza A (H3N2) viruses, and seven (0.5%) were influenza A (H1N1) viruses.
P&I Mortality and ILI Surveillance
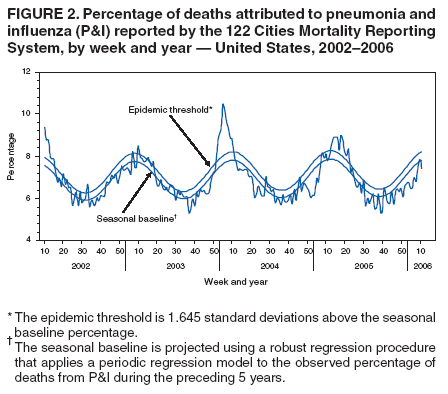
During the week ending January 21, P&I accounted for 7.4% of all deaths reported through the 122 Cities Mortality Reporting System. This percentage is below the epidemic threshold††of 8.2% (See Figure 2).
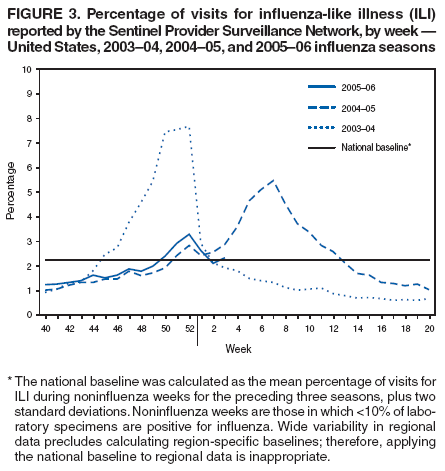
The percentage of patient visits for ILI was 2.3%, which is above the national baseline of 2.2% (See Figure 3). The percentage of patient visits for ILI ranged from 0.9% in the New England region to 6.0% in the West South Central region.
Pediatric Deaths and Hospitalizations
During October 2, 2005–January 21, 2006, CDC received reports of 11 influenza-associated deaths in U.S. residents aged <18 years. Nine of the deaths occurred during the current influenza season, and two occurred during the 2004–05 influenza season.
During October 1, 2005–January 7, 2006, the preliminary influenza-associated hospitalization rate reported by the Emerging Infections Program§§ (EIP) for children aged 0–17 years was 0.18 per 10,000. For children aged 0–4 years and 5–17 years, the rate was 0.48 per 10,000 and 0.02 per 10,000, respectively. During October 30, 2005–January 7, 2006, the New Vaccine Surveillance Network¶¶ (NVSN) reported no laboratory-confirmed influenza-associated hospitalizations among children aged 0–4 years. EIP and NVSN hospitalization rate estimates are preliminary.
Human Avian Influenza A (H5N1)
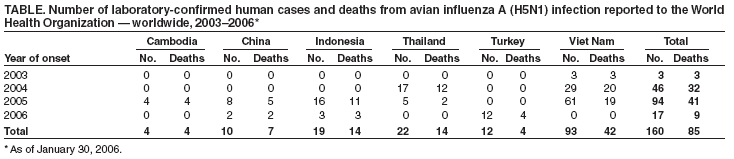
No human avian influenza A (H5N1) virus infection has ever been identified in the United States. From December 2003 through January 30, 2006, a total of 160 laboratory-confirmed human avian influenza A (H5N1) infections were reported to WHO from Cambodia, China, Indonesia, Thailand, Turkey, and Viet Nam.*** Of these, 85 (53%) were fatal (See Table). This represents an increase of one case and one death in China and eight cases and two deaths in Turkey since January 23, 2006. The majority of infections appear to have been acquired from direct contact with infected poultry. No evidence of sustained human-to-human transmission of H5N1 has been detected, although rare instances of human-to-human transmission likely have occurred (1).
Reference
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005;352:333–40.
Notes
* Provisional data reported as of January 27. Additional information about influenza activity is updated each Friday and is available from CDC at http://www.cdc.gov/flu.
†Levels of activity are 1) widespread: outbreaks of influenza or increases in influenza-like illness (ILI) cases and recent laboratory-confirmed influenza in at least half the regions of a state; 2) regional: outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in at least two but less than half the regions of a state; 3) local: outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in a single region of a state; 4) sporadic: small numbers of laboratory-confirmed influenza cases or a single influenza outbreak reported but no increase in cases of ILI; and 5) no activity.
§ Widespread: Colorado, Connecticut, Kansas, Texas, and Wyoming; regional: Alaska, Arizona, Arkansas, California, Georgia, Idaho, Iowa, Kentucky, Maryland, Minnesota, Mississippi, Nebraska, New Mexico, New York, North Carolina, Oregon, Pennsylvania, Rhode Island, Tennessee, Utah, Virginia, Washington, and Wisconsin; local: Florida, Indiana, Massachusetts, Michigan, Montana, Nevada, Ohio, Oklahoma, and South Dakota; sporadic: Alabama, Delaware, Hawaii, Illinois, Louisiana, Maine, Missouri, New Hampshire, New Jersey, North Dakota, South Carolina, Vermont, and West Virginia; no activity: none; no report: none.
¶ Temperature of >100.0°F (>37.8°C) and cough and/or sore throat in the absence of a known cause other than influenza.
** The national baseline was calculated as the mean percentage of visits for ILI during noninfluenza weeks for the preceding three seasons, plus two standard deviations. Noninfluenza weeks are those in which <10% of laboratory specimens are positive for influenza. Wide variability in regional data precludes calculating region-specific baselines; therefore, applying the national baseline to regional data is inappropriate.
††The expected seasonal baseline proportion of P&I deaths reported by the 122 Cities Mortality Reporting System is projected using a robust regression procedure in which a periodic regression model is applied to the observed percentage of deaths from P&I that occurred during the preceding 5 years. The epidemic threshold is 1.645 standard deviations above the seasonal baseline.
§§ The Emerging Infections Program (EIP) Influenza Project conducts surveillance in 60 counties associated with 12 metropolitan areas: San Francisco, California; Denver, Colorado; New Haven, Connecticut; Atlanta, Georgia; Baltimore, Maryland; Minneapolis/St. Paul, Minnesota; Albuquerque, New Mexico; Las Cruces, New Mexico; Albany, New York; Rochester, New York; Portland, Oregon; and Nashville, Tennessee.
¶¶ The New Vaccine Surveillance Network (NVSN) conducts surveillance in Monroe County, New York; Hamilton County, Ohio; and Davidson County, Tennessee.
*** Available at http://www.who.int/csr/disease/avian_influenza/en.