Kenapa Harus Unduh Aplikasi Hp777?
Aplikasi Hp777 menghadirkan pengalaman bermain game terbaik di genggamanmu! 💯 Dengan unduhan gratis, kamu bisa menikmati 50+ game seru seperti slot, tembak ikan, dan kartu tradisional. Fitur unggulan: keamanan berlapis, desain responsif, dan proses transaksi instan. Jackpot progresif siap membuatmu jadi jutawan dalam sekejap! 🚀
Game Terpopuler di Hp777
🔥 Cash Mania (Jackpot: Rp925 Juta): Slot bertema kekayaan dengan bonus putaran gratis dan multiplier hingga 100x! Raih kemenangan beruntun dengan fitur Cash Respin. 💸 🎯 Tembak Ikan (Jackpot: Rp9,8 Miliar): Game arcade laut dalam dengan senjata upgradable. Tembak ikan langka untuk bonus 500x taruhan! 🐠 🏮 Gates of Olympus 1000 (Jackpot: Rp5,9 Miliar): Hadapi dewa Zeus dalam slot megaways dengan 100.000+ cara menang. Fitur tumbling reels bikin kemenangan beruntun! ⚡
Cara Mudah Unduh & Instal
📲 Untuk pengguna Android: 1. Buka Settings > Security > Aktifkan 'Unknown Sources' 2. Kunjungi situs resmi Hp777 3. Klik tombol 'Unduh APK' 4. Instal file setelah download selesai 🍏 Untuk pengguna iOS: 1. Download TestFlight dari App Store 2. Daftar melalui link unduhan resmi Hp777 3. Ikuti petunjuk instalasi 💡 Tips: Dapatkan bonus Rp50.000 setelah instalasi pertama dengan kode: WELCOME777
Fitur Eksklusif Member Hp777
✨ Nikmati keuntungan sebagai member setia: - Bonus Harian 10% (maks Rp100.000) - Cashback Mingguan hingga 15% - Turnamen Bulanan dengan hadiah total Rp500 Juta 🏆 - VIP Program dengan personal account manager - Prioritas withdraw <3 menit ⏱️ 🎁 Event spesial setiap bulan seperti Lucky Spin dan Grand Tournament!
Keamanan & Layanan 24/7
🔒 Hp777 menggunakan enkripsi SSL 256-bit dan sistem firewall canggih. Semua game telah tersertifikasi RNG (Random Number Generator) untuk keadilan mutlak. Tim customer service siap membantu via live chat 24 jam dalam 3 bahasa (Indonesia, Inggris, Mandarin). 💬 📞 Butuh bantuan? Hubungi: - WA: +628123456777 - Email: [email protected] - Live Chat: Langsung di aplikasi
Requirements (Latest version)
- 5.0+ or higher required
Information about unduh aplikasi Hp777 v5.4.1
Rate this App
























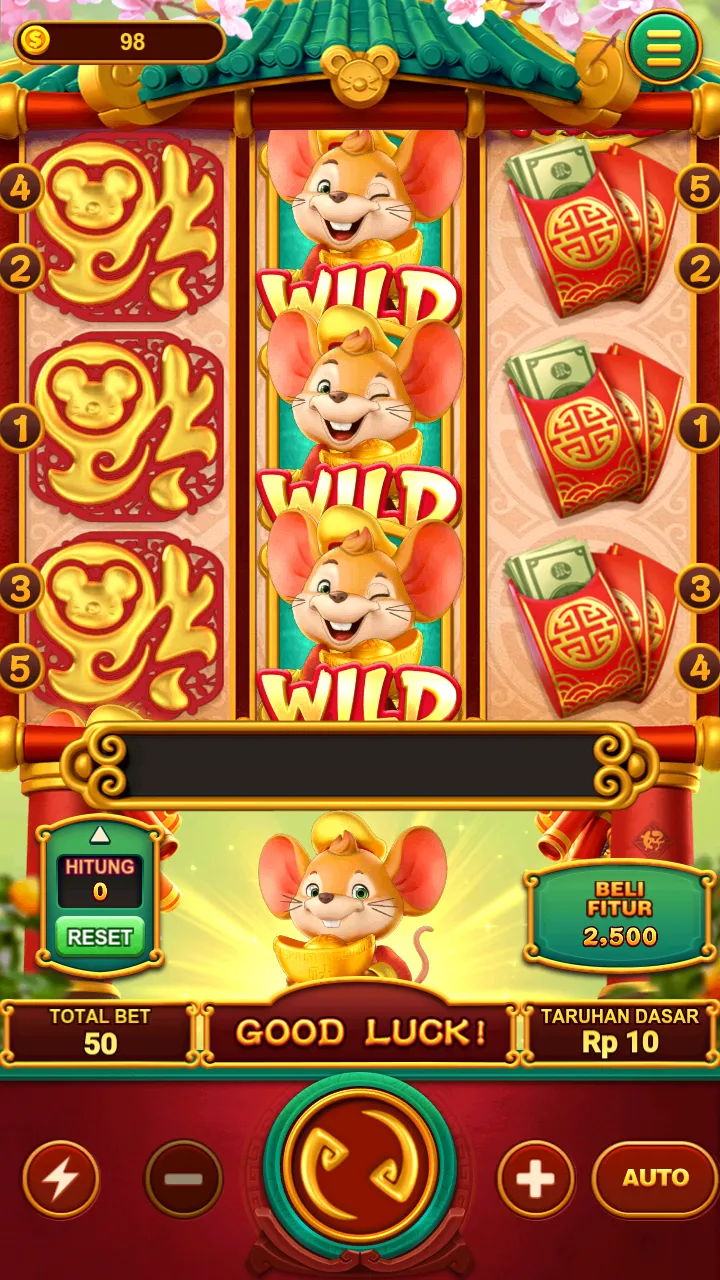





















Comments
Baru seminggu main di Hp777 udah dapet jackpot Rp3,2 juta! Aplikasinya ringan banget di hp lama saya 👍
Withdraw cuma 2 menit cair!! 💸 Gak pernah nemu aplikasi secepat ini, bonus new member-nya juga beneran dikasih
Graphic game Tembak Ikannya keren banget, kayak console game! Tapi gak bikin hp panas. Recommended buat dicoba 🎯
CS-nya responsif banget pas saya lupa password, cuma 5 menit udah bisa login lagi. Salut sama pelayanannya! 👏
Jackpot Gates of Olympus beneran ada! Baru main 3 hari langsung bawa pulang Rp17 juta 🤑 Gak nyangka!
Awalnya ragu, ternyata aplikasinya legal & aman. Proses verifikasi akun cepet banget, cuma 10 menit udah aktif
Turnamen bulanannya seru banget! Meskipun cuma peringkat 27 masih dapet hadiah Rp750 ribu. Worth it banget 💯
Cocok buat yang suka game tradisional kayak Gaple & QiuQiu. Fitur private table-nya bikin main sama temen makin asik!